Clinical Trial Monitoring
Phase I-IV clinical trial expertise
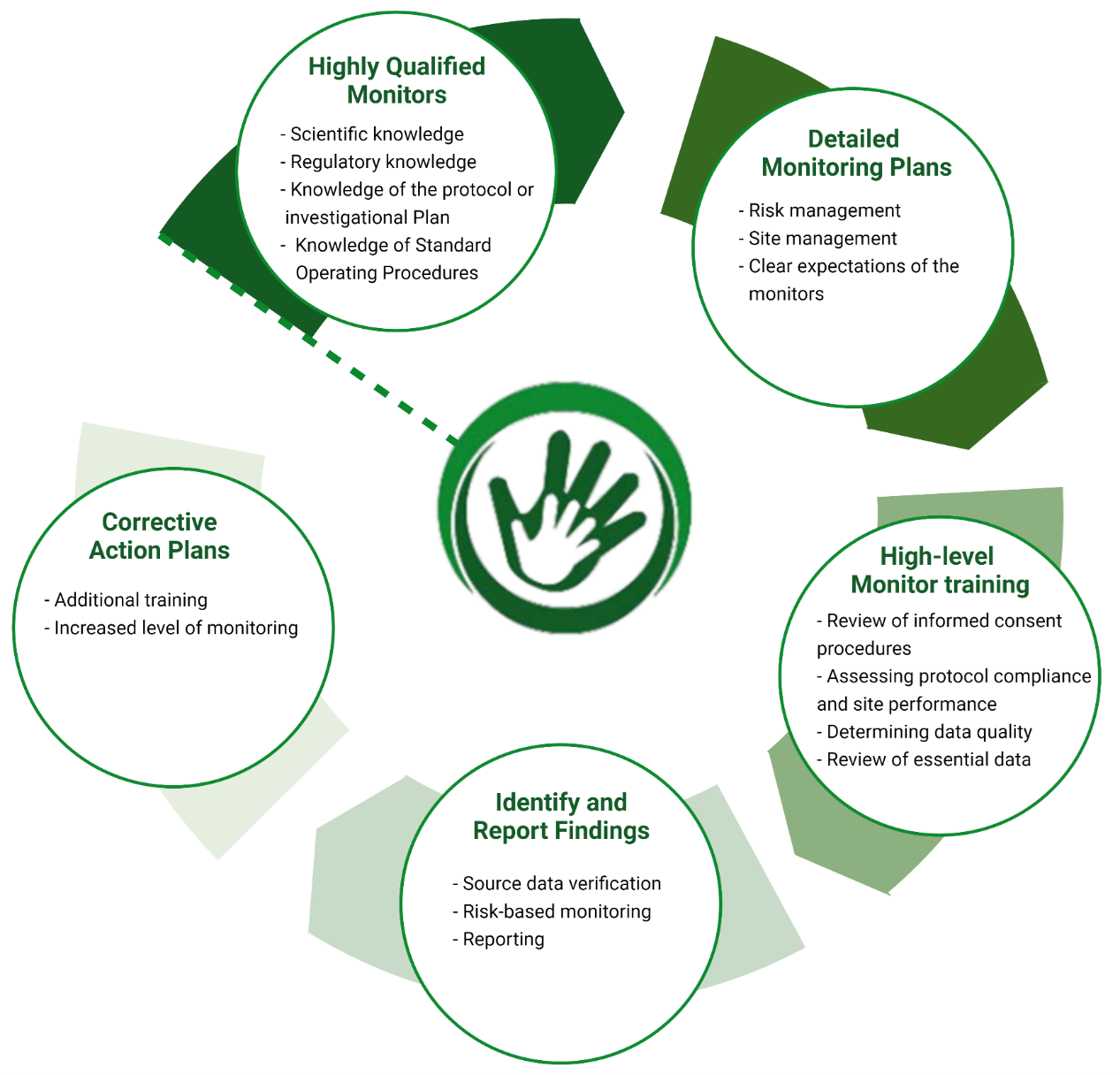
We have a proven track record for delivering the highest quality Clinical Trial Monitoring Services, complying with the applicable regulation and requirements.
Our extensive experience in clinical trial monitoring allows us to effectively oversee the progression of a clinical study and ensure that the trial is conducted, recorded and reported in accordance with the clinical investigational plan, agreements, Good Clinical Practice, requirements of the institutional Review Board (IRB) / Ethics Committee (EC) and all applicable regulation.
We provide customised assistance, closely collaborating with the Sponsor’s Project Management Team. We ensure that we continue our high-level training in diverse therapeutic areas, clinical trial methodologies (decentralised trials, eConsent, ePRO etc) to be able to deliver the highest quality monitoring services and support for trial-sites and vendor staff.